Remdesivir, Rick Bright - the Government Bureaucrat Who Brought Us "Run Death is Near"
In 2020 or 2021, Was Robert Malone warning us about Remdesivir?
Trump’s Coronavirus Task Force was established on January 29, 2020 with Alex Azar as its founding Chair. He was later replaced by VP Mike Pence (February 26, 2020). In the video (below) Dr. Zelenko confirms the research by
& about the behind-the-scenes sabotaging of Hydroxychloroquine by Rick Bright etal (members of the Anthrax Gang). All roads lead back to the Anthrax Gang as George and Mark Kulacz have been reporting (check out Mark’s Remdesivir playlist here and info on Remdesivir here - and George Webb on Twitter, Substack; Charles Wright’s Remdesivir playlist here ).Remdesivir, is a drug that has cost US taxpayers millions of dollars, however, we don’t get to share from its royalties. Behind the scenes, Rick Bright and associates (who have subsequently blamed Dr. Bright as if he were the lone wolf in this debacle) have caused the death of innocent Americans through the use of Remdesivir and the Covid mRNA transfections. Hydroxychloroquine and Ivermectin would have precluded the use of Remdesivir and the mRNA transfections, but money is more important than American lives in the world of big pharma/bioweapons/medical industrial complex.
A true hero who fought to save the lives of his patients - a real medical doctor (not just a framed certificate on the wall with the letters MD): Dr. Zelenko.
Dr Zelenko Exposes Dr Rick Bright for Sabotaging Early Covid Treatment (Hydroxychloroquine), Killing Masses (Dec 4 2021)(video courtesy of )
Dr Zelenko Exposes How Dr Rick Bright’s Very Bad Move Sabotaged Early Covid Treatment Killing Masses
Writes Steve Berger:
Video on how denial of treatment cost lives and hospitalization. Hydroxychloroquine was only allowed by their legal subterfuge for in hospital use. As Dr. Risch from Yale has explained by the time you get to hospital you have Covid pneumonia which is a completely different disease. Hydroxychloroquine is needed for early outpatient treatment a completely different infection where goal is preventing replication he says.
The denial was all fueled by trump derangement syndrome since he used this drug successfully. You can bet all the pols who got Covid found a way to get this or Ivermectin!!
Adaptive COVID-19 Treatment Trial (ACTT) (Remdesivir Study 2/2020)
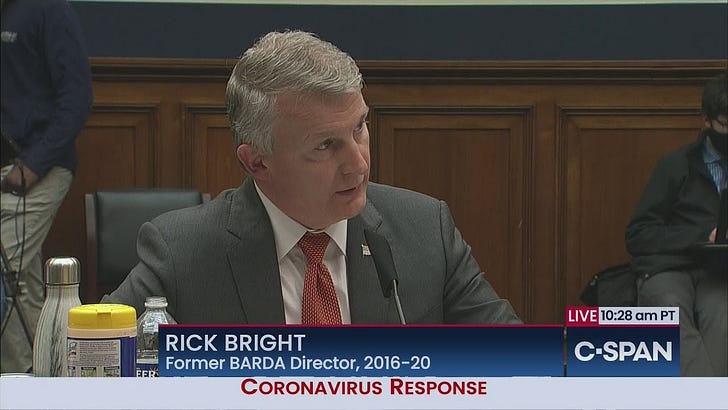
May 14, 2020: Dr. Rick Bright Testifies Before House Energy & Commerce Panel
“33:48 I JOINED BARDA IN 2010, UNTIL APRIL 21 OF THIS YEAR, I HAD THE PRIVILEGE OF SERVING AS ITS DIRECTOR……
“33:48
I JOINED BARDA IN 2010, UNTIL APRIL 21 OF THIS YEAR, I HAD THE
PRIVILEGE OF SERVING AS ITS DIRECTOR. BARDA PARTNERS WITH PRIVATE INDUSTRY TO ADDRESS NATIONAL HEALTH SECURITY THREATS. TODAY THE WORLD IS CONFRONTING A PUBLIC HEALTH EMERGENCY UNLIKE WE HAVE NOT SEEN IN OVER A CENTURY. WE ARE FACING A TRANSMISHL AND DEADLY VIRUS THAT DISRUPTS THE VERY FOUNDATIONS OF OUR SOCIETY. THE AMERICAN HEALTH CARE SYSTEM IS BEING TAXED TO THE LIMIT. OUR ECONOMY IS SPIRALING DOWN-WARD AND OUR POPULATION IS BEING PARALYZED BY FEAR, STEMMING FROM A LACK OF A COORDINATED RESPONSE AND A DAERTH OF ACCURATE, CLEAR INDICATION ABOUT THE PATH FORWARD. AMERICANS YEARN TO GET BACK TO WORK, TO PROVIDE FOR THEIR FAMILIES. I GET THAT. HOWEVER, WHAT WE DO MUST BE DONE FAIRFULLY AND WITH GUIDANCE FROM THE BEST SCIENTIFIC -- CAREFULLY AND WITH GUIDANCE FROM THE BEST SCIENTIFIC MINDS. OUR WINDOW OF OPPORTUNITY IS CLOSING. IF WE FAIL TO IMPROVE OUR RESPONSE NOW, B ON SCIENCE, I FEAR THE PANDEMIC WILL GET WORSE AND BE PROLONGED. THERE WILL LIKELY BE A RESURGENCE OF COVID-19 THIS FALL AND BE GREATLY COMPOUNDED ABOUT THE CHALLENGES OF SEASONAL INFLUENZA. WITHOUT BETTER PLANNING, 2020 COULD BE THE DARKEST WINTER IN MODERN HISTORY. FIRST AND FOREMOST, WE NEED TO BE TRUTHFUL WITH THE AMERICAN PEOPLE. AMERICANS DESERVE THE TRUTH. THE TRUTH MUST BE BASED ON SCIENCE.”
In April 2022, the GAO published this report: SCIENTIFIC INTEGRITY HHS Agencies Need to Develop Procedures and Train Staff on Reporting and Addressing Political Interference. BARDA is a subagency within HHS while DTRA (the “good” agency according to Robert Malone) and DARPA (the “bad” agency according to Robert Malone) are part of the Department of Defense
DARPA, the “bad” agency, according to Robert Malone: “A Pentagon agency known as DARPA is hunting for the three most potent antibodies to combat COVID-19. The Defense Advanced Research Projects Agency set an ambitious goal three years ago, to stop a disease outbreak in just 60 days. Catherine Herridge goes inside the innovative agency as they ready for clinical trials.”
Back to Rick Bright: A month earlier, Dr. Rick Bright spoke on a podcast discussing the successes of BARDA, FDA, CDC and the HHS/NIH during the Covid-19 Plandemic:
April 15, 2020 : Research!America Alliance Member Call with Dr Rick Bright and Scott Whitaker
“as we move aggressively to ensure we're not doing anything bureaucratically that would slow down the potential availability or evaluation of a life-saving drug or diagnostic or vaccine in this outbreak” (Dr. Rick Bright brags about BARDA’s work during Covid-19 plandemic)
“31:02: over the 13 years we've been in existence through our public and private partnerships through industry and collaboration across government this model has yielded 54 new fda approvals licensures and clearance or clearances for everything ranging from influenza pandemic influenza vaccines to ebola vaccines and diagnostics and and therapeutics and chemical medical countermeasures smallpox anthrax a full range for burn products etc of medical countermeasures that have gone over the finish line so they can be used some of these in everyday life to be able to sustain those for when they're needed for an emergency response and some of these are in the strategic national stockpile and procured or acquired so we have those on hand to meet an emergency need when it arises…33:00: one of the mindsets that we are thinking about in BARDA when we stood up a new division of research innovation adventures in 2018 and i think uh Mary Wooley asked a question to Scott a few minutes ago about innovation and where we bring innovation into this whole system you know as we're seeing now in as we look at new drugs and new vaccines and new diagnostics in the in the middle of this response you know the critical need to rethink our development and evaluation and production and distribution processes end to end to make sure that we are not wasting any time making sure that we are thinking strategically and in new ways to be able to make the countermeasures available as quickly as possible we have begun evaluating and in this response we are actually implementing many new strategies to make the medical accounting measures available or get data from medical county majors as quickly as possible and that is only done in collaboration as scott mentioned to the excellent work that the fda has been doing in this response and working with industry partners of all sizes to be able to move as quickly as possible and rethink or revise even some particular strategies if they potentially slowed down a process the entire time keeping safety first and forefront of everyone's mind as we move aggressively to ensure we're not doing anything bureaucratically that would slow down the potential availability or evaluation of a life-saving drug or diagnostic or vaccine in this outbreak so in this slide it just talks about the various areas either a situational awareness this is the diagnostic or testing or the test to characterize whether a person is infected or has already been affected and may be immune and able to re-enter the workforce to how quickly we design a new vaccine or a drug and manufacture and validate those validation as you'll see as we get more and more into the drug development the vaccine development a lot of innovation even in the clinical trial design and regulatory review that we all have to put in place to move as quickly as possible manufacturing will be done in very unique um collaborative ways and and as Scott was talking in his his overview a minute ago talking about the public sector and the private sector and how public and private sector are working more closely in this outbreak it's also really gratifying to see how industry partners are collaborating and teaming in unique ways that i've never seen in my many years in this business putting profit aside in many cases putting competition aside in many cases to bring together the best capabilities to work together to to truly have one focus and mission and that is to end this pandemic as quickly as possible”
Remdesivir: a double-edge sword, partially financed by US taxpayers:
Between 1980 and 2019, the NIH contributed over 2,200 fiscal years of grant funding for research underlying Remdesivir's drug target, RdRp, particularly viral research.
(Government Accountability Office) Biomedical Research:Information on Federal Contributions to Remdesivir GAO Found (the GAO reporting doesn’t date back to 1991)
Between 2013 and 2020, the Centers for Disease Control and Prevention (CDC), the Department of Defense (DOD), and the National Institutes of Health (NIH) conducted and funded preclinical research collaborations with Gilead Sciences, Inc. (Gilead) that helped to demonstrate remdesivir's antiviral properties against multiple viruses. NIH also funded three clinical trials. (See figure for examples of federal support.) Between 2009 and 2013, Gilead had synthesized the remdesivir compound, conducted and funded preclinical research that first identified and confirmed the antiviral activity of remdesivir and its parent compound against coronaviruses and other viruses, and had begun patenting the compounds. As of December 2020, federal funding for preclinical studies and clinical trials involving remdesivir totaled about $162 million (March 31, 2021)
Other GAO Reports that mention Remdesivir (info provided for individuals interested in doing further research on Remdesivir):
Science, Technology Assessment, and Analytics at GAO: September 2022 Update
Review of DOD strategy:
Review of Operation Warp Speed
(Rick Bright and Anthony Fauci were not happy, they wanted to force Remdesivir on us for a few years. They’re on video telling us the vaccines could not be manufactured within timeline that Trump stated):
Know who else financed Remdesivir studies? Yes, the rich tech guru who has funded both Robert Malone and Robert F. Kennedy Jr. (started a SUPER PAC for Kennedy, has paid for Malone interviews, what’s in it for him?)- Steve Kirsch!
The ‘very, very bad look' of Remdesivir, the first FDA-approved COVID-19 drug
The Food and Drug Administration held no advisory meeting on antiviral, and the European Union signed contract without knowing of failed trial
The World Health Organization's (WHO's) Solidarity trial showed that remdesivir does not reduce mortality or the time COVID-19 patients take to recover. (10/28/2020)
“October was a good month for Gilead Sciences, the giant manufacturer of antivirals headquartered in Foster City, California. On 8 October, the company inked an agreement to supply the European Union with its drug remdesivir as a treatment for COVID-19—a deal potentially worth more than $1 billion. Two weeks later, on 22 October, the U.S. Food and Drug Administration (FDA) approved remdesivir for use against the pandemic coronavirus SARS-CoV-2 in the United States—the first drug to receive that status. The EU and U.S. decisions pave the way for Gilead's drug into two major markets, both with soaring COVID-19 cases.
But both decisions baffled scientists who have closely watched the clinical trials of remdesivir unfold over the past 6 months—and who have many questions about remdesivir's worth. At best, one large, well-designed study found remdesivir modestly reduced the time to recover from COVID-19 in hospitalized patients with severe illness. A few smaller studies found no impact of treatment on the disease whatsoever. Then, on 15 October—in this month's decidedly unfavorable news for Gilead—the fourth and largest controlled study delivered what some believed was a coup de grâce: The World Health Organization's (WHO's) Solidarity trial showed that remdesivir does not reduce mortality or the time COVID-19 patients take to recover.”
Folks in the back, can y’all get up and shout out “All Roads Lead Back to the Anthrax Gang?”
WHO: Remdesivir shouldn't be used for patients hospitalized with COVID 19
11/21/2020
Multi-site Adaptive Trials for COVID-19 (Famotidine, Northwell Health)
On 29 April 2020, the National Institute of Allergy and Infectious Diseases (NIAID) announced that Remdesivir was better than placebo in reducing time to recovery for people hospitalized with advanced COVID-19 and lung involvement. In an earlier study of adult patients admitted to a hospital for severe COVID-19, Remdesivir was not associated with statistically significant clinical benefits. In that study, Remdesivir was not associated with a difference in time to clinical improvement. Although not statistically significant, patients receiving Remdesivir had a numerically faster time to clinical improvement than those receiving placebo among patients with symptom duration of 10 days or less. Remdesivir was stopped early because of higher numbers of adverse events compared to placebo. Because of these studies the FDA stated on 1 May 2020, that it is "reasonable to believe" that known and potential benefits of Remdesivir outweigh its known and potential risks, in some specific populations hospitalized with severe COVID-19.
Hospital/treatment guidelines (search for “Remdesivir” within document):
207.2: TrialSiteNews Mar 2020 highlights most prescient CV19 prediction: Sina Bavari 2018 Remdesivir
Manufacturing (of Remdesivir (data from Housatonic.Live))
Remdesivir requires "70 raw materials, reagents, and catalysts" to make, and approximately "25 chemical steps."[47] Some of the ingredients are extremely dangerous to humans, especially trimethylsilyl cyanide.[47] The original end-to-end manufacturing process required 9 to 12 months to go from raw materials at contract manufacturers to finished product, but after restarting production in January 2020, Gilead Sciences was able to find ways to reduce the production time to six months.[47]
In January 2020, Gilead began working on restarting remdesivir production in glass-lined steel chemical reactors at its manufacturing plant in Edmonton, Alberta.[47] On 2 February 2020, the company flew its entire stock of remdesivir, 100 kilograms in powder form (left over from Ebola research), to its filling plant in La Verne, California to start filling vials.[47] The Edmonton plant finished its first new batch of remdesivir in April 2020.[47] Around the same time, fresh raw materials began to arrive from contract manufacturers reactivated by Gilead in January.[47]
Another challenge is getting remdesivir into patients despite the drug's "poor predicted solubility and poor stability."[48] In June 2020, Ligand Pharmaceuticals revealed that Gilead has been managing those issues by mixing Ligand's proprietary excipient Captisol (based on University of Kansas research into cyclodextrin) with remdesivir at a 30:1 ratio.[48] Since that implies an enormous amount of Captisol is needed to stabilize and deliver remdesivir (on top of amounts needed for several other drugs for which the excipient is already in regular use), Ligand announced that it is trying to boost Captisol annual manufacturing capacity to as much as 500 metric tons.[48]
Foundational research and NIH funding enabling Emergency Use Authorization of Remdesivir for COVID-19 (from medrxiv.org)
“Emergency Use Authorization for remdesivir months after discovery of COVID-19 is unprecedented. Typically, decades of research and public-sector funding are required to establish the mature body of foundational research requisite for efficient, targeted drug discovery and development. This work quantifies the body of research related to remdesivir’s biological target, RNA-dependent RNA polymerase (RdRp), or parent chemical structure, nucleoside analogs (NcAn), through 2019, as well as NIH funding for this research 2000–2019.
SIGNIFICANCE STATEMENT
Emergency Use Authorization of remdesivir for treating COVID-19 four months after discovery of this virus was enabled by decades of research on the drug’s biological target as well as other medicines with related chemical structures. The NIH contributed 6,800 years of grant funding to this research, totaling $6.5 billion (2000–2019), including funding for both investigator-initiated research and research infrastructure. Of this, $46.5 million was for research directly related to remdesivir. This analysis demonstrates the importance of a robust body of foundational research in responding rapidly to emergent diseases, and the substantial NIH contribution to this research. It also underscores the scale and significance of the public-sector investments that enable new drug discovery and development.
Topic Focus of NIH-Funded RdRp and NcAn Research. The large majority of NIH-funded publications on RdRp (89%) mentioned the term virus or viral, with the majority accounted for by research related to Hepatitis, influenza, Coronavirus, Zika, and Ebola virus (Figure 2A), while 19% contained the wild card term “immun*” and 1.3% mentioned cancer.”
Timeline (United States) (Covid19/Remdesivir from Housatonic.Live)
In March 2020, United States President Donald Trump announced that remdesivir was available for "compassionate use" for people with COVID‑19; FDA Commissioner Stephen Hahn confirmed the statement at the same press conference.[80] It was later revealed that Gilead had been providing remdesivir in response to compassionate use requests since 25 January.[19][81] On 23 March 2020, Gilead voluntarily suspended access for compassionate use (excepting cases of critically ill children and pregnant women), for reasons related to supply, citing the need to continue to provide the agent for testing in clinical trials.[82][83]
In May 2020, the US Food and Drug Administration granted Gilead emergency use authorization (EUA) for remdesivir to be distributed and used by licensed health care providers to treat adults and children hospitalized with severe COVID‑19.[57][31] Severe COVID‑19 is defined as patients with an oxygen saturation (SpO2) <= 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO), a heart–lung bypass machine.[12][31][84][85] Distribution of remdesivir under the EUA was controlled by the US government for use consistent with the terms and conditions of the EUA.[31] Gilead supplied remdesivir to authorized distributors, or directly to a US government agency, who distributed it to hospitals and other healthcare facilities as directed by the US government, in collaboration with state and local government authorities, as needed.[31] Gilead stated they were donating 1.5 million vials for emergency use[84] and estimated, as of April 2020, they had enough remdesivir for 140,000 treatment courses and expect to have 500,000 courses by October 2020, and one million courses by the end of 2020.[86][87]
The initial distribution of the drug in the US was tripped up by seemingly capricious decision-making and finger-pointing, resulting in over a week of confusion and frustration among health care providers and patients alike.[88][89][90] On 9 May 2020, the United States Department of Health and Human Services (HHS) explained in a statement that it would be distributing remdesivir vials to state health departments, then would allow each department to redistribute vials to hospitals in their respective states based upon each department's insight into "community-level needs."[91] HHS also clarified that only 607,000 vials of Gilead's promised donation of 1.5 million vials would be going to American patients.[91] However, HHS did not explain why several states with some of the highest caseloads had been omitted from the first two distribution rounds, including California, Florida, and Pennsylvania.[91] In May 2020, Gilead indicated they would increase the number of doses donated to the US from 607,000 to around 940,000.[92][90] Some of the initial distribution was sent to the wrong hospitals, to hospitals with no intensive care units, and to facilities without the needed refrigeration to store it.[90]
In June 2020, HHS announced an unusual agreement with Gilead in which HHS agreed to Gilead's wholesale acquisition price, HHS would continue to work together with state governments and drug wholesaler AmerisourceBergen to allocate shipments of remdesivir vials to American hospitals through the end of September 2020, and in exchange, during that three-month timeframe (July, August, and September), American patients would be allocated over 90% of Gilead's projected remdesivir output of more than 500,000 treatment courses.[93][94] Absent from these announcements was any discussion of allocation of remdesivir production to the approximately 70 countries omitted from Gilead's generic drug licensing agreements—including much of Europe[95] and countries as populous as Brazil, China, and Mexico—or the 127 countries listed on those agreements (during the time it will take for Gilead's generic licensees to ramp up their own production).[96] As the implications of this began to sink in, several countries publicly confirmed the next day that they already had adequate supplies of remdesivir to cover current needs, including Australia,[97] Germany,[98] and the United Kingdom.[99]
In August 2020, the FDA broadened the Emergency Use Authorization (EUA) for remdesivir to include all hospitalized patients with suspected or laboratory-confirmed COVID‑19, irrespective of the severity of their disease.[100][101] The Fact Sheet was updated to reflect the new guidance.[12]
In October 2020, Gilead and HHS announced that HHS was relinquishing control over remdesivir allocation because production of the drug had finally caught up with US domestic demand.[102][103][104] AmerisourceBergen will remain the sole distributor of Veklury in the US through the end of 2020.[104]
On 22 October 2020, the FDA approved remdesivir and also revised the EUA to permit the use of remdesivir for treatment of suspected or laboratory confirmed COVID‑19 in hospitalized children weighing 3.5 kilograms (7.7 lb) to less than 40 kilograms (88 lb) or hospitalized children less than twelve years of age weighing at least 3.5 kilograms (7.7 lb).[24][12][105][106] This decision was criticized for an alleged lack of previous consultation on part of the FDA given the complications of antiviral drug issues.[107]
In November 2020, the FDA issued an EUA for the combination of baricitinib with remdesivir, for the treatment of suspected or laboratory-confirmed COVID‑19 in hospitalized people two years of age or older requiring supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).[28] The data supporting the EUA for baricitinib combined with remdesivir are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), which was conducted by the National Institute of Allergy and Infectious Diseases (NIAID).[28] The EUA was issued to Eli Lilly and Company.[28]
Remdesivir received approval from the US Food and Drug Administration (FDA) in October 2020, for use in adults and children twelve years and older requiring hospitalization for treatment of severe COVID‑19 infections.[24] In January 2022, the FDA gave regulatory approval to remdesivir for use in adults and children (twelve years of age and older who weigh at least 40 kilograms (88 lb) and are positive for COVID‑19, not hospitalized, and are ill with COVID‑19 having high risk for developing severe COVID‑19, including hospitalization or death.[25]
The FDA also provided Emergency Use Authorization in 2022, for remdesivir treatment of children under age twelve who are COVID-positive and not hospitalized, but have mild-to-moderate COVID‑19 with high risk of developing severe infection, including hospitalization or death.[25]
Folks have been making some noise about the outrageous amount of cash being thrown at the biomedical research industry - the Federal Government is responding:
(Government Accountability Office) Better Data Will Improve Understanding of Federal Contributions to Drug Development
The National Institutes of Health—the largest public funder of biomedical research in the U.S.—invests billions of dollars each year to help develop new drugs or new uses for existing drugs. But the extent of NIH's contributions to drug development isn't well understood or recognized by the public.
One reason may be that recipients of NIH funds aren't always disclosing agency support fully or correctly when applying for patents.
We recommended that NIH clarify that its awardees should name NIH as the funding agency. This will help the public and policymakers better understand how NIH's investments translate into drugs that benefit Americans. (April 4, 2023)
Which agency spent the most money (FYE 2020) on Research and Development?
Back to Rick Bright….
Reviewing Rick Bright starting Remdesivir orders/pipeline before any trials:
Ep 179.2: Remdesivir timeline Review #2 - Baric not inventor, Bright Everywhere, XIANGGUO QIU ZMAPP (Housatonic.Live)
“25:09…as we'll see as we go forward in the timeline here Rick Bright actually wound up being one of them the top promoters of Remdesivir in the entire United States government”
Back to Right Bright’s “whistleblower” theatrical act:
Rick Bright is rewarded by the US Government for being a “whistleblower” not “whistleblower” when he was demoted for insubordination. His participation on the video conference call in April 2020, tells us a different story - he was bragging about the COVID response. Can we get our money back?
OSC Announces Settlement Agreement Between HHS and Former BARDA Director Dr. Rick Bright After his Reassignment
8/9/2021
PROHIBITED PERSONNEL PRACTICES
The U.S. Office of Special Counsel (OSC) today announced a settlement agreement reached between the U.S. Department of Health and Human Services (HHS) and Dr. Rick Bright, the former Director of the Biomedical Advanced Research and Development Authority (BARDA).
While serving as BARDA director, Dr. Bright disclosed potential safety risks and the lack of efficacy associated with use of chloroquine and hydroxychloroquine as therapeutic treatments for COVID-19. Dr. Bright also disclosed concerns about the government's pandemic response and about influence exerted by political and industrial interests on BARDA and HHS, both before and during the pandemic. After Dr. Bright's disclosures, HHS reassigned Dr. Bright to a non-supervisory position at the National Institutes of Health.
Dr. Bright filed a prohibited personnel practice complaint with OSC alleging, among other issues, that the reassignment limiting his role in the government's pandemic response was retaliatory. Consistent with its standard practice, OSC conducted an investigation of Dr. Bright's allegations, and also helped encourage and facilitate a mutually agreeable resolution. Ultimately, the parties were able to settle the case, resolving Dr. Bright's whistleblower retaliation allegations.
“I am pleased that HHS and Dr. Bright were able to reach a settlement and move forward on mutually agreeable terms," said Special Counsel Henry J. Kerner. “OSC takes seriously its role in investigating allegations of prohibited personnel practices. A settlement in this matter is a good outcome and a testament to OSC staff's diligent work in helping facilitate a resolution."
March 2021: Rick Bright joins the Rockefeller Foundation:
Unlisted video with superstar not superstar Rick Bright on The Rockefeller Foundation Youtube channel:
October 2022: The rock star couldn’t bring home the bacon (unlike his longtime buddy, who has negotiated multimillion dollar contracts/funding):
“Q: It sounds like you weren’t meeting the goals that you had set for attracting outside funding?
A: That is accurate. It was really difficult to raise the funds to support this institute. Maybe we started a year too late. If we’d started sooner, I think it’d be much easier to attract those matching funds, because you were still seeing this growing threat [from COVID-19]. Look at how hard it is to get Congress to make funds available for the work we still need to do for this pandemic, let alone preparing for the next one. And around the world, it’s the same.”
When public health specialist Rick Bright launched the Pandemic Prevention Institute (PPI) under the aegis of the Rockefeller Foundation last year, he recognized that several other efforts—some old, some new—had similarly ambitious visions for how to make the world safer from pathogens. “No one can do it all,” Bright told Science in October 2021, when the institute was 7 months old. “We have to now come together to decide how we divide and conquer this ecosystem.”
But as Bloomberg revealed on 26 September, Rockefeller now has unexpectedly decided to break apart PPI, to which it had pledged $150 million over 3 years. In particular, it will fold some of PPI’s work into the foundation’s increasing efforts on climate change, which many scientists argue will increase outbreaks of novel threats by altering the movements of pathogen-carrying animals and increasing their interactions with humans. And as a result of this strategic shift, Bright is leaving Rockefeller.
Bright has had a high-profile career and, before joining Rockefeller, became embroiled in a controversy that made headlines. For 5 years, Bright headed the U.S. Biomedical Advanced Research and Development Authority (BARDA), which had a $1 billion budget to fund diagnostics, treatments, and prevention tools like vaccines and masks. Battles with his boss about how to best use BARDA funds and criticism of former President Donald Trump’s scientifically unfounded push to use hydroxychloroquine as a COVID-19 treatment led to his resignation, a whistleblower suit, testimony at a congressional hearing, and a segment on 60 Minutes.
Q: When you took the job, you and the foundation knew there were all these other entities doing similar work. So it can’t come as a surprise to Rockefeller leadership that you were one of many fish in the sea.
A: I don’t think it’s necessarily just the fact that there are other people doing it. I was just in Belfast for the 70th anniversary of the WHO’s [World Health Organization’s] Global Influenza Surveillance and Response System. When you take a deep look at that network of networks, where they have reference labs, regulatory labs, and national Influenza centers in over 158 countries, they share information, new tools, and knowledge, and they have that 70 years of history and trust. Compare that to a new entity that wants to enter the landscape: The amount of time and effort it would take to build that trust is hard to calculate. I’m confident we can make technologies that work and can connect the data more efficiently and in new ways. But we can’t buy that network of trust built up over those years.
Q: My guess is that it isn’t your decision to disband PPI.
A: It is not my decision.
Q: So would you have kept it going?
A: After working with thousands of companies when I was at BARDA, you do see some that come through with a great idea and they just keep pushing it and it goes. They really start working in the first year or two, making progress, but then it seems to slow, and there are diminishing returns. My mindset has always been to succeed early, and if you’re not going to hit your goals and have the big impact you thought you would, pivot your strategy early. So what I really appreciate with the Rockefeller Foundation is I felt like we went all in. We had space, we had people, we had money, we had partners, we had the reputation and name. Rockefeller gave this a really good go.
Then the smart thing to do is look at the long-term business strategy. How many hundreds of millions or billions of dollars would it take to really build this fully and sustain it? It became clear that it was going to be much more difficult than we initially anticipated. So I followed my philosophy to pivot early. I’m confident it’s the right decision. When I look at the networks that are already in place, it almost sets the wrong tone, and can be more of a distraction or a disruption, to try to tell the world that the organizations and institutes that we have are hopeless and that we need to do something completely different and build a separate institute.
Q: It sounds like you weren’t meeting the goals that you had set for attracting outside funding?
A: That is accurate. It was really difficult to raise the funds to support this institute. Maybe we started a year too late. If we’d started sooner, I think it’d be much easier to attract those matching funds, because you were still seeing this growing threat [from COVID-19]. Look at how hard it is to get Congress to make funds available for the work we still need to do for this pandemic, let alone preparing for the next one. And around the world, it’s the same.
Q: What happens to the people you brought on as staff?
A: It’s my understanding that everyone on our team is being folded into the larger health and climate strategy. I’m hoping that the work that we’ve done is not ending. The thing that’s really ending is the concept of spinning out a whole separate free-standing institute.
Q: What’s next for Rick Bright?
A: I will continue working in pandemic preparedness response. I plan to advise organizations that are working on their strategic plans, working on their budget requests, doing what they need to do to get the funding necessary to implement. My next big act, I don’t know exactly what it will be. I have a lot of opportunities—my phone is ringing off the hook and emails are filling up. But I’m going to take some time. I am disheartened seeing the tidal waves going back out to sea at this point, the funding going back out.
Q: Any interest in returning to work for the U.S. government?
A: For the right position, where I could have impact in shaping strategies and making sure we can secure the funding and resources necessary to implement those strategies, not only for the U.S., but for the world? I would entertain that.
The Rockefeller Foundation Pandemic Institute still exists and thrives, without the rockstar.
Back to the killing of Hydroxychloroquine:
The coordinated killing of Trump’s request to make Hydroxychloroquine available to all Americans was allegedly coordinated behind the scenes by bad hombres, led by the rockstar not rockstar Rick Bright.
Why was an obscure federal bureaucrat (Rick Bright) involved in Trump’s emergency hydroxychloroquine authorization?
Why did the FDA issue an EUA in the first place?
HHS says it needed an EUA for hydroxychloroquine and chloroquine because the agency had received millions of donated doses of unapproved versions of the drug. (Similar versions, from multiple manufacturers, are FDA approved, but only to treat other conditions). Because the donated drugs were not FDA approved, HHS needed legal authority to put the drugs into the national stockpile, the U.S. government’s vast supply of emergency drugs and devices kept on hand to respond to pandemics.
There is some debate as to whether the government needed to issue an EUA to accept the donation of the drugs. Outside observers, like Borio and Goodman, for example, have argued that the government could have instead facilitated access to these drugs through FDA’s so-called compassionate use program, a separate government system set up to try to help dying patients get access to experimental treatments that haven’t yet been approved.
But the government was facing an unusual situation, to be sure: HHS was hoping to distribute doses of a drug that the FDA hadn’t technically approved — even though the agency had approved an identical product, made by other manufacturers. And HHS wasn’t just hoping to get the drugs to the American people, like a compassionate use program might do. It also wanted to add them to the national stockpile, so, an EUA likely was the quickest and most legally sound method for doing so.
Why did Bright’s name appear on the EUA?
It’s certainly not typical for an EUA to bear the name of the head of the Biomedical Advanced Research and Development Authority, the post Bright held before he was demoted. Typically, these requests come from companies directly or from the director of the CDC, which often makes its own tests to detect infectious diseases that then need emergency authorization to be used. There have also been instances in years past of CDC requesting EUAs for products being added to the national stockpile.
In fact, after reviewing more than 100 emergency authorizations issued by the FDA since 2009, STAT could not find another instance of a BARDA director directly requesting one.
But BARDA, an office under HHS, does play a key role in overseeing products added to the national stockpile, so it makes some sense that the request might come from him.
BARDA and the FDA also have a long standing agreement, known as a memorandum of understanding, that allows the two offices to talk about the approval of products that help solve public health emergencies. (FDA officials are otherwise barred from discussing unapproved drugs.) So, BARDA is not entirely removed from the process of securing regulatory approval for products like hydroxychloroquine.
BARDA and its parent office, the Assistant Secretary for Preparedness and Response, have actually taken on a larger role overseeing the stockpile in recent years: The stockpile was moved out of CDC control to ASPR control in 2018, making it all the more logical that someone at ASPR, like Bright, would request the emergency authorization, rather than the CDC director.
HOW NIH HOODWINKED THE COUNTRY ABOUT THE NEED FOR COVID VACCINES: DRUG COMPANY THERAPIES AND THE NEED FOR GOVERNMENT OVERSIGHT (8/27/2021)
Drug companies have antiviral drugs coming soon for early COVID. Merck’s Molnupiravir was given a $1.2 billion contract. At $750 a treatment, it needs to be compared to ivermectin at $5 a treatment and Regeneron antibodies at $2100 a treatment. Pfizer has an oral protease inhibitor coming. The General Accountability Office has been tasked with this along with estimating the economic damage caused by the failure of NIH to do anything with repurposed drugs. The SEC has been tasked with investigating the multiple New York Stock Exchange traded companies that have been involved in trying to hide ivermectin from COVID infected patients. Many of their investors would not approve. It has been brought to the attention of the House Committee on Oversight and Reform who has been investigating drug company overpricing. Information about the deceptive actions of our government healthcare agencies in regards to repurposed drugs was faxed to the offices of 5 democratic US senators 3 months ago and .
Blast from the past - the Vaccine Frankensteins pushing their vision of fast vaccine manufacturing, new technology at the WHO….
World Health Organization: Vaccine Production Strategies: Ensuring Alignment and Sustainability
“12-14 July 2011 - The World Health Organization (WHO) hosted the second Consultation on the Global Action Plan for Influenza Vaccines to review the progress for the first time since it was developed in 2006.
More than 100 representatives attended the meeting to review the progress on the key objectives of the plan and to develop a strategic plan of action for the next five years. The main focus of the second consultation was to discuss countries' experience on pandemic preparedness and vaccine production.
Invited speaker: Robert W Malone, MD, MS”
Rapid response, rapid vaccines….unless Donald Trump wants them manufactured rapidly.
Watch til the end - whose soft, feminine, wispy voice is speaking to Robert Malone? No, it’s not Robert Malone’s wife. (video from Jill Malone’s Vimeo channel, published here for educational/informational purposes, link to original video is above)
It’s Rick Bright, the same government parasite who installed his longtime buddy, Robert Malone, as the leader of the Famotidine study.
Rick Bright and Robert Malone have a long history of working together, no one should be surprised that Bright brought in Malone to “manage” the Famotidine clinical trial taking place at Northwell Hospital. Malone ended up quitting, citing, once again, a difficult work environment.
Rick Bright, Robert Malone and the Famotidine Clinical Trials……
New York clinical trial quietly tests heartburn remedy against coronavirus
Hospital data from China and a classified drug screening program led to the rapid launch of $21 million study (04/26/2020)
NOT SO FAST, DR. KEVIN TRACEY, YOU THINK YOU LEAD THE FAMOTIDINE STUDY, BUT RICK BRIGHT INSTALLED ROBERT MALONE AS THE BOSS OF THIS CONTRACT…(“On March 20, Kadlec wrote to Dr. Kevin Tracey, Northwell’s executive vice president for research. He instructed Tracey to work with Callahan to prepare a contract proposal and a draft budget for the Pepcid trial.”)
(On August 16, 2020 both Dr. Kevin Tracey and Dr. Michael Callahan published a report on Famotidine: Famotidine Use Is Associated With Improved Clinical Outcomes in Hospitalized COVID-19 Patients: A Propensity Score Matched Retrospective Cohort Study, pdf found below).
The study was written on behalf of the Famotidine Research Group.
“Members of the Famotidine Research Group: Magdalena E. Sobieszczyk, MD, MPH (Division of Infectious Diseases, Columbia University Irving Medical Center-New York Presbyterian Hospital, New York, New York), David D. Markowitz, MD (Division of Digestive and Liver Diseases, Columbia University Irving Medical Center-New York Presbyterian Hospital, New York, New York), Aakriti Gupta, MD, MS (Division of Cardiology, Columbia University Irving Medical Center-New York Presbyterian Hospital, New York, New York), Max R. O’Donnell, MD, MPH (Division of Pulmonary, Allergy, and Critical Care Medicine, Columbia University Irving Medical Center-New York Presbyterian Hospital, New York, New York), Jianhua Li, MD (Department of Medicine, Columbia University Irving Medical Center-New York Presbyterian Hospital, New York, New York), David A. Tuveson, MD, PhD (Cancer Center, Cold Spring Harbor Laboratory, Cold Spring Harbor, New York), Zhezhen Jin, PhD (Department of Biostatistics, Columbia University Mailman School of Public Health, New York, New York), William C. Turner, MD (Department of Medicine, Columbia University Irving Medical Center-New York Presbyterian Hospital, New York, New York), and Donald W. Landry, MD, PhD (Department of Medicine, Columbia University Irving Medical Center-New York Presbyterian Hospital, New York, New York)”
“On 14 April, the U.S. Biomedical Advanced Research and Development Authority (BARDA) (RICK BRIGHT), which operates under Kadlec, gave Alchem (ROBERT MALONE) a $20.7 million contract for the trial, most of which paid Northwell's costs.”
WHY WOULD RICK BRIGHT INSTALL ROBERT MALONE AS LEADER OF A TRIAL THAT NORTHWELL WAS HANDLING? (“On March 20, Kadlec wrote to Dr. Kevin Tracey, Northwell’s executive vice president for research. He instructed Tracey to work with Callahan to prepare a contract proposal and a draft budget for the Pepcid trial.”)
“On 7 April, the first COVID-19 patients at Northwell Health in the New York City area began to receive famotidine intravenously, at nine times the heartburn dose. Unlike other drugs the 23-hospital system is testing, including Regeneron's sarilumab and Gilead Sciences's remdesivir, Northwell kept the famotidine study under wraps to secure a research stockpile before other hospitals, or even the federal government, started to buy it. "If we talked about this to the wrong people or too soon, the drug supply would be gone," says Kevin Tracey, a former neurosurgeon in charge of the hospital system's research.
A globe-trotting infectious disease doctor named Michael Callahan was the first to call attention to the drug in the United States. Callahan, who is based at Massachusetts General Hospital and has extensive connections in the biodefense world, has spent time in disease hot zones around the world, including the 2003 outbreak of another coronavirus disease, SARS, in Hong Kong. In mid-January, he was in Nanjing, China, working on an avian flu project. As the COVID-19 epidemic began to explode in Wuhan, he followed his Chinese colleagues to the increasingly desperate city.
In reviewing 6212 COVID-19 patient records, the doctors noticed that many survivors had been suffering from chronic heartburn and were on famotidine rather than more-expensive omeprazole (Prilosec), the medicine of choice both in the United States and among wealthier Chinese. Hospitalized COVID-19 patients on famotidine appeared to be dying at a rate of about 14% compared with 27% for those not on the drug, although the analysis was crude and the result was not statistically significant.
But that was enough for Callahan to pursue the issue back home. After returning from Wuhan, he briefed Robert Kadlec, assistant secretary for preparedness and response at the Department of Health and Human Services, then checked in with Robert Malone, chief medical officer of Florida-based Alchem Laboratories, a contract manufacturing organization. Malone is part of a classified project called DOMANE that uses computer simulations, artificial intelligence, and other methods to rapidly identify U.S. Food and Drug Administration (FDA)-approved drugs and other safe compounds that can be repurposed against threats such as new viruses.
Malone had his eyes on a viral enzyme called the papainlike protease, which helps the pathogen replicate. To see whether famotidine binds to the protein, he would ordinarily need the enzyme's 3D structure, but that would not be available for months. So Malone recruited computational chemist Joshua Pottel, president of Montreal-based Molecular Forecaster, to predict it from two crystal structures of the protease from the 2003 SARS coronavirus, combined with the new coronavirus' RNA sequence.
It was hardly plug-and-play. Among other things, they compared the gene sequences of the new and old proteases to rule out crucial differences in structure. Pottel then tested how 2600 different compounds interact with the new protease. The modeling yielded several dozen promising hits that pharmaceutical chemists and other experts narrowed to three. Famotidine was one. (The compound has not popped up in in vitro screens of existing drug libraries for antiviral activity, however.)
After getting FDA approval, Northwell used its own funds to launch the effort. Just getting half of the needed famotidine in sterile vials took weeks, because the injectable version is not widely used. On 14 April, the U.S. Biomedical Advanced Research and Development Authority (BARDA), which operates under Kadlec, gave Alchem a $20.7 million contract for the trial, most of which paid Northwell's costs.
The study's draft protocol was aimed only at evaluating famotidine's efficacy, but Trump's "game-changer" antimalarial drug was rapidly becoming the standard of care for hospitalized COVID-19 patients. That meant investigators would only be able to recruit enough subjects for a trial that tested a combination of famotidine and hydroxychloroquine. Those patients would be compared with a hydroxychloroquine-only arm and a historic control arm made up of hundreds of patients treated earlier in the outbreak. "Is it good science? No," Tracey says. "It's the real world."
Pepcid as a virus remedy? Trump admin’s $21M gamble fizzled
“Peter Navarro, Trump’s top assistant for trade and manufacturing policy, said in a March 19 email that he would soon be “flooding” Kadlec’s office with contracts “and I cannot have these kind of bullshit delays at HHS.”
“Navarro didn’t specify which contracts and there’s no indication he advocated famotidine as a COVID-19 treatment.
“Your shop is now officially a bottleneck,” Navarro told Kadlec.
Kadlec took action the next day, according to internal government correspondence. He contacted a longtime colleague of Callahan’s at Northwell Health, New York state’s largest health care provider, to request the expedited clinical trial of famotidine and the anti-malarial drug hydroxychloroquine. Trump had been touting hydroxychloroquine though it too was unproven against COVID-19. Its emergency use would later be revoked by the FDA amid growing evidence the drug failed at treating the disease and could cause serious side effects.
On March 20, Kadlec wrote to Dr. Kevin Tracey, Northwell’s executive vice president for research. He instructed Tracey to work with Callahan to prepare a contract proposal and a draft budget for the Pepcid trial.
Federal pandemic response scientists at the Biomedical Advanced Research and Development Authority, or BARDA, were shut out of these early conversations about famotidine. Rick Bright, BARDA’s director at the time, would later file a whistleblower complaint alleging unethical conduct by agency leadership, and point to the Pepcid trial as a key example.”
On March 31, Northwell’s Tracey emailed Kadlec. He said Callahan had been assisting and that Northwell had designed “a fully implementable trial under emergency status.” Azar, the administration’s top-ranking health official, and his assistant secretary for health, Admiral Brett Giroir, were copied on the message. Giroir also is the admiral who leads the U.S. Public Health Service Commissioned Corps.
“It is my understanding that ADM Giroir and Secretary Azar have been briefed and express interest in supporting a randomized clinical trial to determine the safety and potential efficacy for the use of famotidine in COVID-19,” Tracey wrote in the email to Kadlec.
The FDA roadblock lifted suddenly. Northwell agreed to restrict the duration of the IV doses to 14 days, and the FDA’s Murray wrote in an April 2 email the trial could move forward. Northwell Health spokesman Matthew Libassi described the dialogue between with the FDA as the normal back and forth when starting a new clinical trial.
“In January, Kadlec, who for years had traveled in the same biodefense circles as Callahan, tapped the doctor as a key adviser.
“…the doctors who initially promoted the Pepcid idea are locked in a battle for credit and sniping over allegations of scientific misconduct.”
“Dr. Robert Malone said he got a call on Jan. 4 from Michael Callahan, a fellow American doctor working in China, according to Malone’s own written summary and an interview with AP. The doctor told Malone -- a molecular virologist who was chief medical officer of the Florida-based pharmaceutical company Alchem Laboratories -- about a new coronavirus-like disease outbreak in Wuhan, the provincial capital of China’s Hubei province.
Malone, a prolific social media poster who raises a rare breed of Portuguese horses on a farm in Virginia, also serves as a consultant to a Pentagon-funded program that develops medications to protect American troops from biological threats. Malone said he recognized such a threat in the pathogen tearing through Wuhan. The virus was moving so fast that there did not appear to be enough time to develop a vaccine.
Malone and a team of volunteers began looking for existing drugs that might be useful. About a week after the call about Wuhan, Chinese scientists published the virus’ genetic fingerprint. Malone ran the sequence through computer models designed to find already-approved drugs that might work to thwart the virus.
One of the most promising leads was famotidine, he said.
By late February, Malone was convinced of famotidine’s safety and efficacy as a COVID-19 drug -- so much so that, when he contracted the disease, he took the drug himself. He reported on his LinkedIn page that he’d figured out the proper dose and became “the first to take the drug to treat my own case.”
But Callahan says it was he, not Malone, who first recognized famotidine’s potential. Callahan is a well-connected infectious disease expert at Massachusetts General Hospital and an adviser to Dr. Robert Kadlec, a retired Air Force colonel who is assistant secretary for preparedness and response at the Department of Health and Human Services. Kadlec’s job is to help guide the country through public health emergencies.
When the virus hit in late 2019, Callahan was already in Wuhan working with Chinese infectious disease researchers. Callahan said he and the Chinese doctors analyzed the medical records of more than 6,000 hospitalized patients, 1,100 of whom had severe COVID-19 disease, according to information released by researchers conducting the clinical trial.
Callahan did not respond to requests from The AP for comment, but his account is detailed in promotional materials about a potential Pepcid trial.
About 600 of the severely ill Chinese COVID-19 patients were on famotidine antacids and their disease was found to be milder than others of similar age and health.
Callahan hasn’t published or made public any data to back up the Wuhan account. But his credentials and recent experience in China were enough for Kadlec. In January, Kadlec, who for years had traveled in the same biodefense circles as Callahan, tapped the doctor as a key adviser.”
“Kadlec had only Callahan’s anecdotal experience with the heartburn drug in Wuhan, and Malone’s computer modeling results which indicated famotidine might work against the virus.” (Folks in the back, can you yell “DOMANE”?)
“And Northwell’s work with Pepcid was at too early a stage to provide meaningful data for a trial. The health care provider had just begun drafting plans for studying famotidine when Kadlec contacted them, according to dates provided by Northwell. Within days, Northwell would team up with Malone’s employer, Alchem Laboratories, to study the heartburn drug’s potential as a COVID-19 therapy.
On March 31, Northwell’s Tracey emailed Kadlec. He said Callahan had been assisting and that Northwell had designed “a fully implementable trial under emergency status.””
““We stepped in to do it on behalf of Northwell (which) knows nothing about federal contracting,” Malone told The AP. (but wait….. Northwell Direct to provide telehealth services to U.S. Department of State)
“This initial budget document was a confusing spreadsheet and sought about $250,000 -- a paltry amount compared to the $20.7 million eventually allotted for the contract.” (How much did Malone net from the grossly inflated $20.7 million contract?)
Northwell spokesman Libassi disputed that Malone took control of preparing the contract proposal and said he became “difficult to work with” as the plans for trial progressed.”
“With FDA’s approval to move forward, Northwell sent Kadlec a preliminary trial budget, a copy of which was obtained by The AP. This initial budget document was a confusing spreadsheet and sought about $250,000 -- a paltry amount compared to the $20.7 million eventually allotted for the contract.
Malone said the initial low estimate was Northwell’s mistake, and that the nearly $21 million sum was reached after his team got involved. “We stepped in to do it on behalf of Northwell (which) knows nothing about federal contracting,” Malone told The AP.
Northwell spokesman Libassi disputed that Malone took control of preparing the contract proposal and said he became “difficult to work with” as the plans for trial progressed.”
“In an email, Disbrow, now BARDA’s acting director, called the proposal a “Callahan thing.” (Why does BARDA say it’s a “Callahan thing” if Malone says that he came up with the idea?)
““Can you believe they want to use Pepcid AC now?” Bright quoted his then-deputy, Gary Disbrow, as saying during a phone call between the two. In an email, Disbrow, now BARDA’s acting director, called the proposal a “Callahan thing.” Bright recounted the exchange in the whistleblower complaint he filed in early May with the U.S. Office of Special Counsel.
The standard way for a pandemic-related research project to get funding is through BARDA, and only after rigorous reviews. The Pepcid project took a different route. The study was vetted through a fast-track program created by Kadlec called ASPR Next, which Bright alleges was designed to circumvent BARDA’s scientific review. In the end, though, BARDA’s then-director Bright’s complaint said that he “was entirely excluded by Dr. Kadlec from the award process on this contract” even though the money came from his office’s budget.””
“In the end, though, BARDA’s then-director Bright’s complaint said that he “was entirely excluded by Dr. Kadlec from the award process on this contract” even though the money came from his office’s budget.” (In retaliation, Bright installed his long time buddy, Robert Malone, to “manage” the contract to Northwell Health)
Folks in the back, are you seeing the grift? Was Malone supposed to control Michael Callahan’s actions since Callahan became a key advisor to Kadlec?
“Malone resigned as Alchem’s chief medical officer a week later, citing what he described as a difficult work environment. He has since been critical of Callahan and the project.”
Always the perpetual victim, blaming others and “difficult work environments”…personally, I prefer street fighters that don’t easily give up and don’t bully others to expose their confidential contacts (as told by Mathew Crawford/RTE to his followers and “interview” (take a PEPCID before you watch this video, it’s painful to witness the waffling, Mathew was extremely patient) with ).
“On April 14, the federal government awarded a $20.7 million contract to Malone’s employer, Alchem, and its subcontractor, Northwell, for a trial to assess the safety and effectiveness of “the combination of hydroxychloroquine and famotidine for the treatment of moderate to severe COVID-19 disease,” according to a brief summary of the award.
Malone resigned as Alchem’s chief medical officer a week later, citing what he described as a difficult work environment. He has since been critical of Callahan and the project. Meantime, the trial has been paused indefinitely because of a dearth of new patients in New York.
“The Northwell trial is just a zombie at this point,” Malone said. “Completely irrelevant, except in a negative sense.”
Still, Kadlec said through a spokeswoman he would choose to fund the trial again. “If it could save lives, yes.””
























Looks like https://www.treatearly.org/ is offline. Wonder why?
Wow. Lots to go through here.